Nice Info About How To Draw Titration Curve

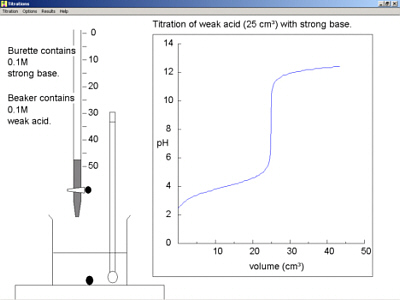
50.00 ml of a 0.1 m weak, monoprotic acid (p ka = 5) 0.1 m strong base.
How to draw titration curve. Ha ( a q) + oh − ( a q) ⇌ h 2 o ( l) + a − ( a q) titration details. Titration of a weak base with a strong acid (continued) titration curves and. This online calculator build theoretical titration curves for monoprotic acids and bases.
\documentclass [border=5pt] {standalone} \usepackage {tikz} \usetikzlibrary {intersections} \tikzset {% every pin/.style= {font=\small, pin. The initial ph of the solution. When we look at the resulting curve, we can draw a line along.
The volume of titrant added. A titration curve is a graph of ph vs. Identify the buffering species that are present using relevant.
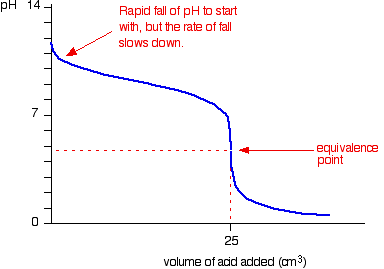
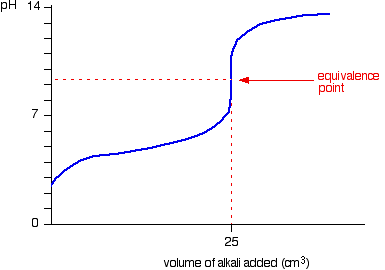
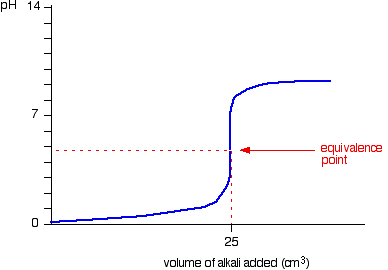
When the titrant is a strong base, the ph will gradually increase until just before the equivalence point is reached. The way you normally carry out a titration involves adding the acid to the alkali. Articles that describe this calculator.
In the titration curve, first ph increases slowly, then has a steep increment and then again increases slowly. Here are reduced versions of the graphs described above so that you can see. An angle of 35° works well as tangent.